This article was created by EcoMundo. EcoMundo is a renowned specialist in regulatory compliance services (REACH, CLP, Cosmetics, Biocides, etc.) and SaaS solutions for regulatory chemical management (FDS, chemical risk, regulatory watch, etc.). EcoMundo helps cosmetic companies throughout the compliance processes of their products all over the world.
For many actors of the European cosmetics & personal care products industry, the Cosmetic Regulation n°1223/2009 is the reference for putting compliant and safe products on the European market. Putting a cosmetic product on the European market requires having a Responsible Person, creating a Product Information File, having a compliant label, carrying out a CPNP notification.
Discover the 5 key steps, recommended by EcoMundo’s experts, to placing your products on the European market:
Step 1: appointment of a Responsible Person
Step 2: control of the product composition
Step 3: creation of the Product Information File (PIF)
Step 4: creation of compliant labels
Step 5: CPNP notification before placing the product on the market
Step 1: the appointment of a Responsible Person
What is a Responsible Person?
A Responsible Person, or RP, is a natural or legal person established in the European Union acting for all the European countries at once.
Who can be a Responsible Person?
- The manufacturer established in the EU
- The European importer: each importer can take the role of RP if the cosmetic product is manufactured outside the European Union
- The European distributor: the distributor becomes RP if the product is placed on the market under its name or brand, or if he modifies the product already on the market with the risk to affect the product compliance with the Regulation.
- A person established in the Community: the manufacturer or importer can appoint a third person established in the community, e.g. a consulting firm specialized in cosmetic compliance.
In the case where the manufacturer, the importer or the distributor appoint a Responsible Person, the only formalism imposed by Cosmetic Regulation 1223/2009 is that there has to be a written agreement between the parties.
What are your Responsible Person’s responsibilities?
The Responsible Person is:
- The preferred contact point with the authorities; the authorities will turn to the RP to ask for information.
- The product compliance guarantor; the Responsible Person is in charge of keeping the Product Information File for 10 years after the last batch of the product is placed on the market.
- The product safety guarantor; the Responsible Person ensures that the product is safe for human health.
- Lastly, the Responsible Person is in charge of the respect of several obligations, such as:
- Carrying out cosmetovigilance,
- Checking claims substantiation.
How to choose a trustworthy Responsible Person?
The Responsible Person can be a legal or natural person: because of the multiplicity of his/her responsibilities, it is often simpler to call on the services of consulting firms specialized in this field, i.e. cosmetology, toxicology, Pharmacy, etc.
Step 2: the product composition review
The second crucial step to validate before placing your cosmetic products on the market, is to review the composition or formula of your cosmetic product on a regulatory perspective, i.e against the Annexes of the Regulation. Here are some key definitions:
- Cosmetic product: any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odors.
- Cosmetic raw material: mixture of ingredients.
- Cosmetic ingredient: a chemical element and its compounds in the natural state or obtained by any manufacturing process, including any additive necessary to preserve its stability and any impurity deriving from the process used but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition.
- Allergen: Ingredient susceptible to provoke an allergic reaction to certain subjects. Currently there are 26 recognized allergens and those must be labelled if their concentration exceeds the 0.01% threshold in rinse-off cosmetic products, and 0.0001% in leave-on products.
Not all ingredients have the same regulatory status!
Some ingredients are prohibited, restricted or allowed by the Cosmetic Regulation. You should make sure that the product that you want to place on the market is fully compliant with the Annexes of the Regulation outlining these restrictions.
a. Prohibited ingredients
Annex II: These prohibited substances are classified in Annex II of the Regulation 1223/2009.
CMR substances: The CMR substances (Carcinogenic, Mutagen, Reprotoxic) are listed in Annex VI of the CLP Regulation (EC No 1272/2008), and when they are of categories 2, 1A or 1B, they are prohibited.
Nonetheless, they can be authorized in finished cosmetic products under the following conditions:
- If they are compliant with the provisions related to the safety of food products, as defined by Regulation (EU) No 178/2002.
- In case there is no substance for substitution that is appropriate after the analysis of alternative solutions.
- If a request has been made for a particular use of the products’ category, with a determined exposition.
- When the substances have been assessed and judged safe by the SCCS for a use in cosmetic products (sole condition for category CMR 2).
b. Restricted ingredients
These substances are listed in Annex III of the Cosmetic Regulation, which counts about 300 substances. These ingredients can be used in cosmetic products only under the conditions described in the annex (product type, purity criteria, percentage of maximal use, etc.). etc.).
c. Authorized ingredients
Three functions of ingredients are listed by the Regulation: colorants, preservative agents and UV filters.
- Allowed colorants: they are listed in Annex IV of Cosmetic Regulation
- Allowed preservative agents: they are listed in Annex V
- Allowed UV filters: they are listed in Annex VI
These ingredients are authorized if they comply with the Regulation's restrictions and depending on the type of products in which they are contained, the part of the body that is concerned, as well as the concentration of the ingredient in the product.
A simple way to check your cosmetic product composition
To check the compliance of the formula of your cosmetic product, you must compare the ingredients to the Annexes of the Regulation. It is important to note that this work must be done on a regular basis, insofar as these annexes are updated several times a year (on an average of three months). It is crucial to conduct a continuous regulatory watch.
Depending on the complexity of your formulas, you may want to use a software that will make the process quicker and more reliable.
Step 3: creation of the Product Information File (PIF)
Another prerequisite to place your cosmetic product on the EU market is the constitution of the Product Information File (PIF). The PIF has to be made available for the authorities, which can consult it at any time (electronic or paper). The drafting and the keeping of the PIF is mandatory even for cosmetics placed on the market before the entry into force of the Regulation (2013).
What is the PIF?
The PIF (Product Information File) is a regulatory file which must be:
- Kept for 10 years (from the date when the last batch of the cosmetic product is placed on the market) by the Responsible Person.
- Kept under electronic or paper format.
- Written in an easily understandable language for the authorities of the member states where it is archived.
What must the PIF contain?
- A description of the product: the Regulation does not impose a standardized form, but imposes “a description of the cosmetic product which enables the Product Information File to be clearly attributed to the cosmetic product”.
- The Product Safety Report.
- A description of the method of manufacturing and a statement of compliance with the Good Manufacturing Practices.
- The proof of the effect(s) claimed for the cosmetic product.
- Data on any animal testing performed by the manufacturer, its agents or suppliers, related to the development or safety assessment of the cosmetic product or its ingredients, including any animal testing performed to meet the legislative or regulatory requirements of third countries.
a. The product description
This description has to enable the reader to identify the product, without ambiguity, and to establish a clear relation between the cosmetic product and its PIF.
b. The Product Safety Report
This report aims to establish a safety assessment of the cosmetic product on the basis of relevant information. The product safety report must be filled in accordance with Annex I of the Cosmetic Regulation.
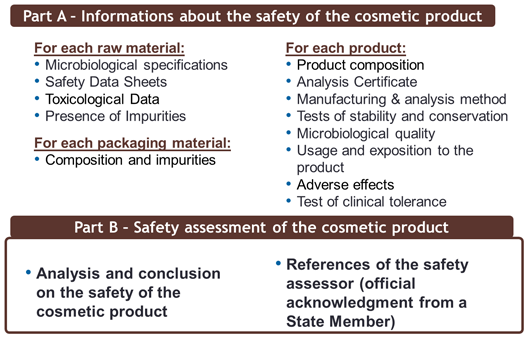
Two distinct parts:
- Part A: Information about the safety of the cosmetic product
- Part B: Safety assessment of the cosmetic product
c. Good Manufacturing Practices (GMP)
The Good Manufacturing Practices come from the ISO standard 22716 published in 2007, providing the guidelines for the production, the storage, and the shipping of cosmetic products.
The PIF must contain a description of the manufacturing conditions and the declaration of compliance with the ISO 22716 Good Manufacturing Practices.
d. Claim substantiation
The Claims must comply with Regulation No 655/2013 specifically drafted for the making of claims.
Claims are texts, names, trade marks, pictures and figurative or other signs shall not be used to imply that these products have characteristics or functions which they do not have. The proofs of the claims may be: consumer perception test, clinical tests, bibliographic search, properties of ingredients that compose the product.
e. Animal testing
Animal experimentation is prohibited: the testing ban on finished products applies since September 2004. The testing ban on ingredients or raw materials applies since March 2009. Also, a marketing ban applies since March 2009 for all human health effects with the exception of repeated-dose toxicity, reproductive toxicity and toxicokinetics for the EU market.
However, all historical animal data can still be used and be relied on for the drafting of cosmetic safety assessment or to fill a data gap for toxicological endpoint such as skin irritation.

Regarding animal experimentation, the PIF must include data related to tests on animals that were carried out by the manufacturer, its staff or its providers.
Step 4: Creating compliant labels
Mandatory requirements on the label:
-
Name and address of the Responsible Person
-
Country of origin
-
Nominal content
-
Date of minimum durability or period-after-opening
-
Precautions & warnings*
-
Batch number
-
Product function*
-
Ingredients list
*Translation in the language of the export country is mandatory. Some countries require full translation of the label, i.e. even the marketing content and claims.
Statements must appear visibly, legibly and indelibly on the label.
Which symbols should I use?
The hourglass symbol to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add date near the symbol
If the DOMD exceeds 30 months, the open-jar symbol will indicate the Period After Opening "PAO” defined by the combination of the stability test and challenge test.
The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with the product with more regulatory information.
Step 5: Notify using the CPNP portal
Last but not least, the Responsible Person must notify the European Commission via the CPNP portal, a website that Responsible Persons and Authorities can use to access products’ information. Regulation No 1223/2009 requires that any company willing to place a cosmetic product on the market must notify online before doing so.
What must be transmitted at the time of notification?
- Name and category of the product.
- The name and address of the Responsible Person, plus contact details.
- In case of import, the country of origin.
- The first country where the product will be placed on the market.
- The presence of nanomaterials and CMR substances.
- The cosmetic product formulation.
- A compliant label with photo of the external packaging (if legible).
Only one notification is needed for all 31 countries of the European Economic Area (EEA).
This blog post was originally published on EcoMondo
Disclaimer:The information provided (on our blog) is accurate to the best of our knowledge, however, there may be errors. As a neutral organization, we at Covalo do not advocate or promote certain products or ingredients on our platform as better than others. The Site may contain (or you may be sent through the Site) links to other websites or content belonging to or originating from third parties or links to websites and features in banners or other advertising. Such external links are not investigated, monitored, or checked for accuracy, adequacy, validity, reliability, availability or completeness by us. For more information on our blog, contact social@covalo.com


